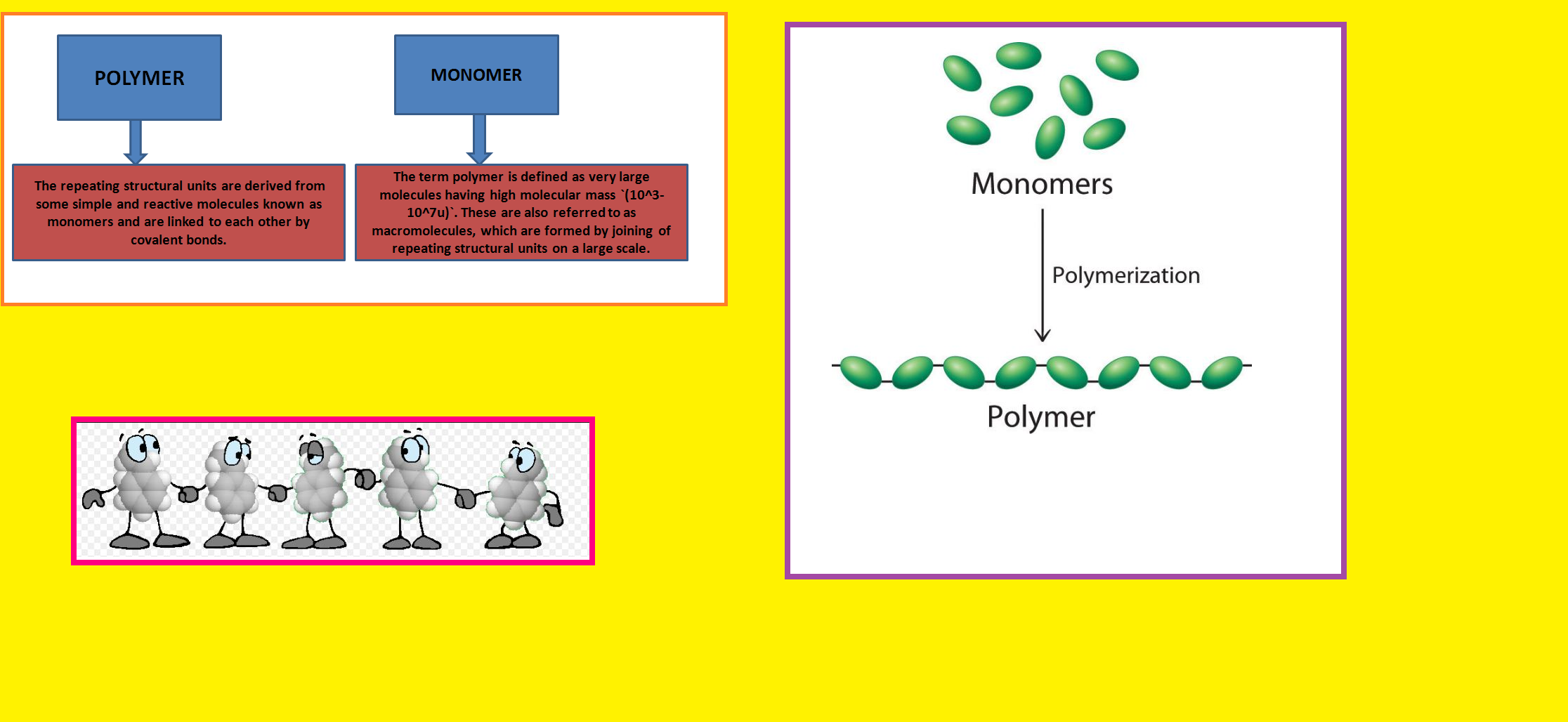
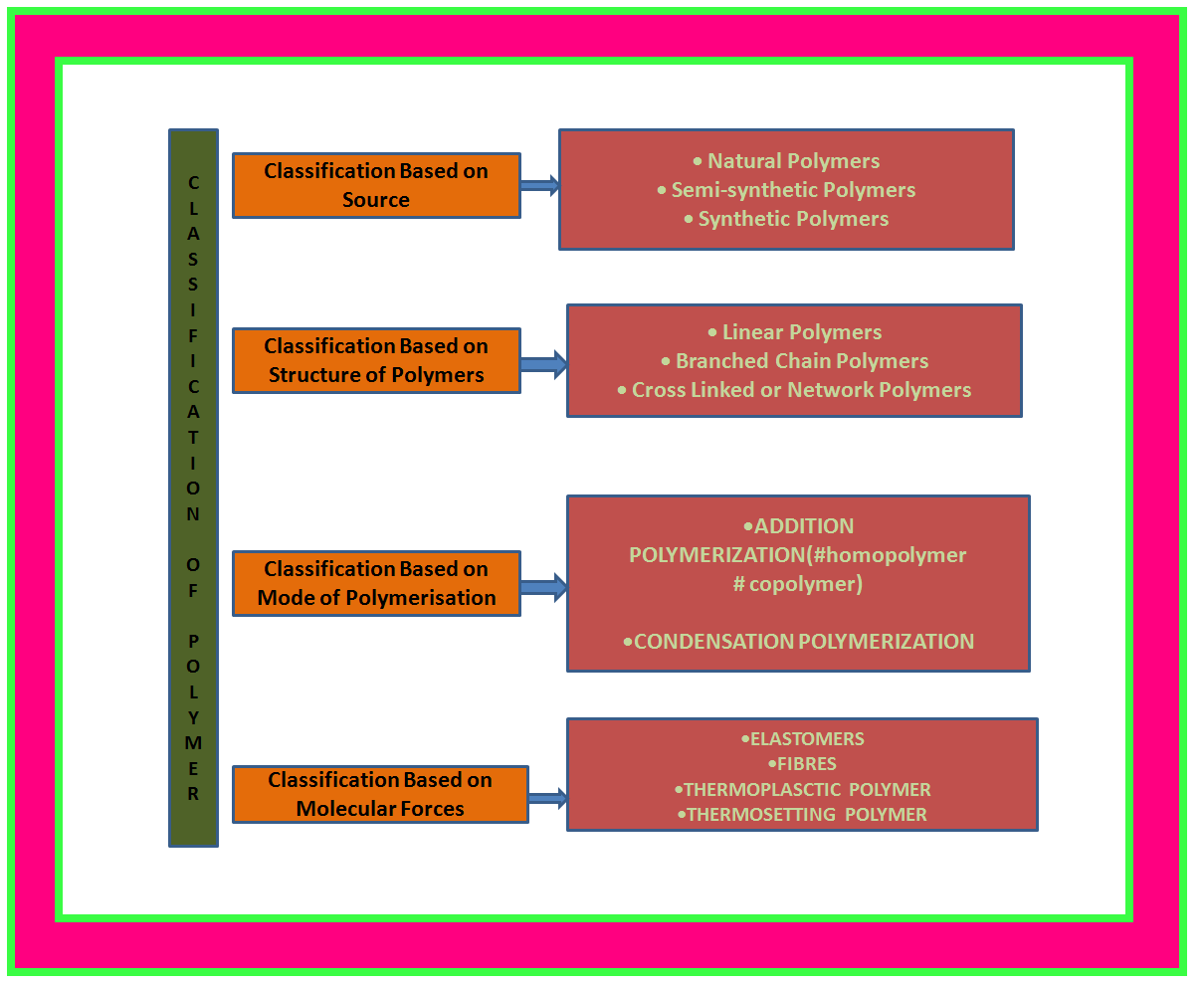
• `color{green}("Natural Polymers ")` : These polymers are found in plants and animals. Examples are proteins, cellulose, starch, resins and rubber.
• `color{green}("Semi-synthetic Polymers ")` : Cellulose derivatives as cellulose acetate (rayon) and cellulose nitrate, etc. are the usual examples of this sub category.
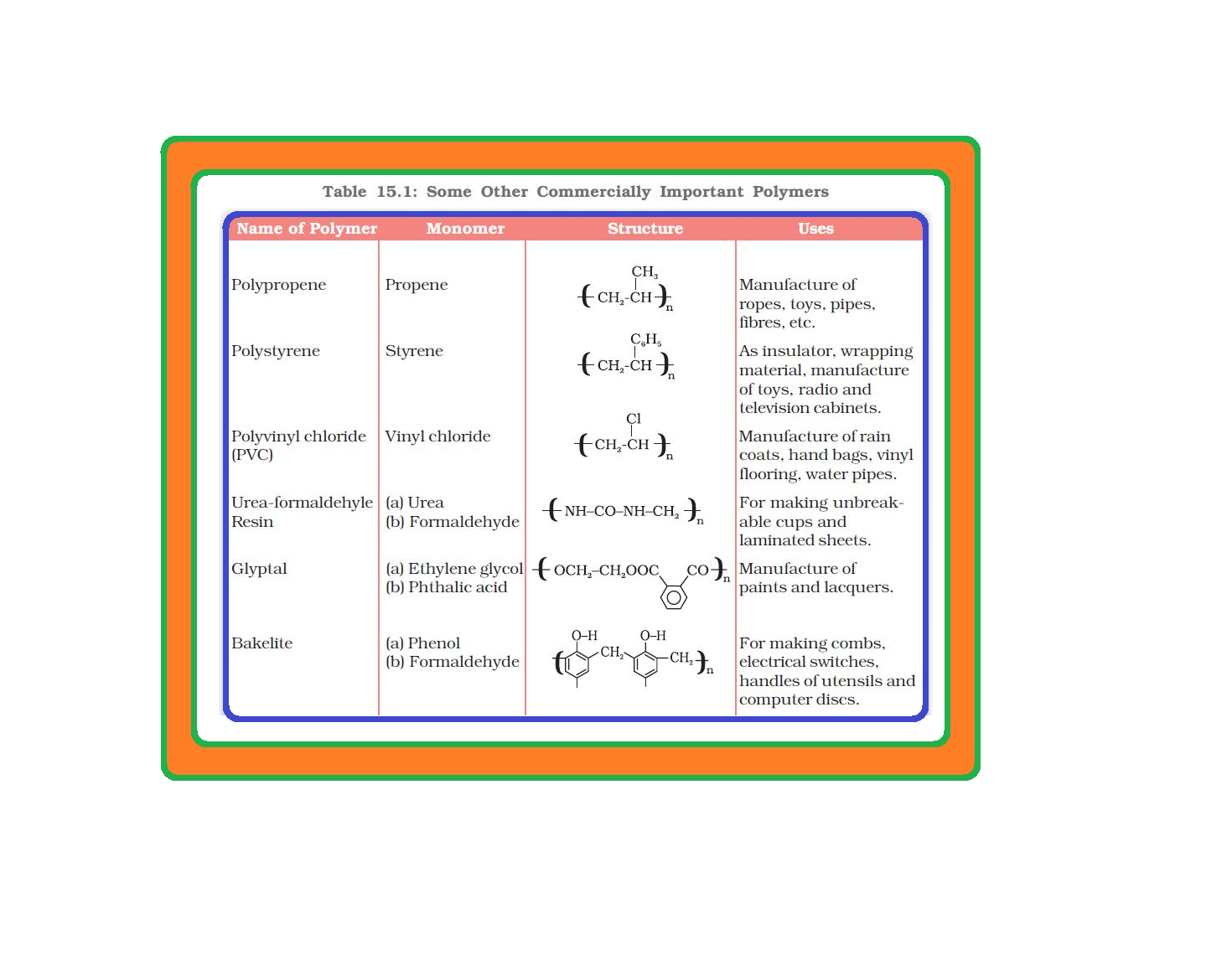
• `color{green}("Synthetic Polymers ")` A variety of synthetic polymers as plastic (polythene), synthetic fibres (nylon 6, 6) and synthetic rubbers (Buna-S) are examples of man made polymers extensively used in daily life as well as in industry.
•`color{green}("Linear Polymers ")` : These polymers consist of long and straight chains. The examples are high density polythene, polyvinyl chloride, etc. These are represented as shown in fig.1.
•`color{green}("Branched Chain Polymers ")` : These polymers contain linear chains having some branches, e.g. low density polythene.
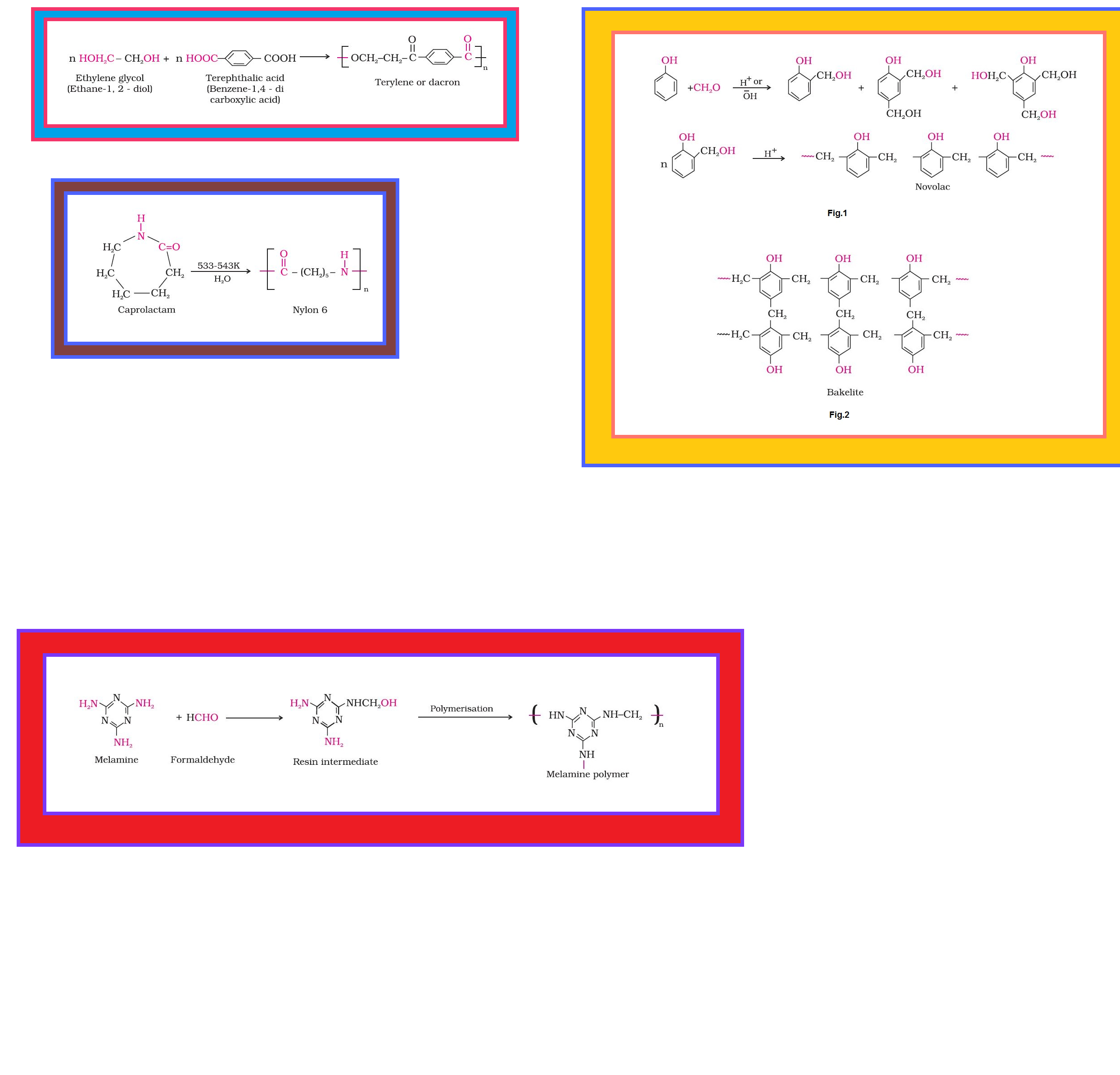
•`color{green}("Cross Linked or Network Polymers ")` : These are usually formed from bi-functional and tri-functional monomers and contain strong covalent bonds between various linear polymer chains, e.g. bakelite, melamine, etc.
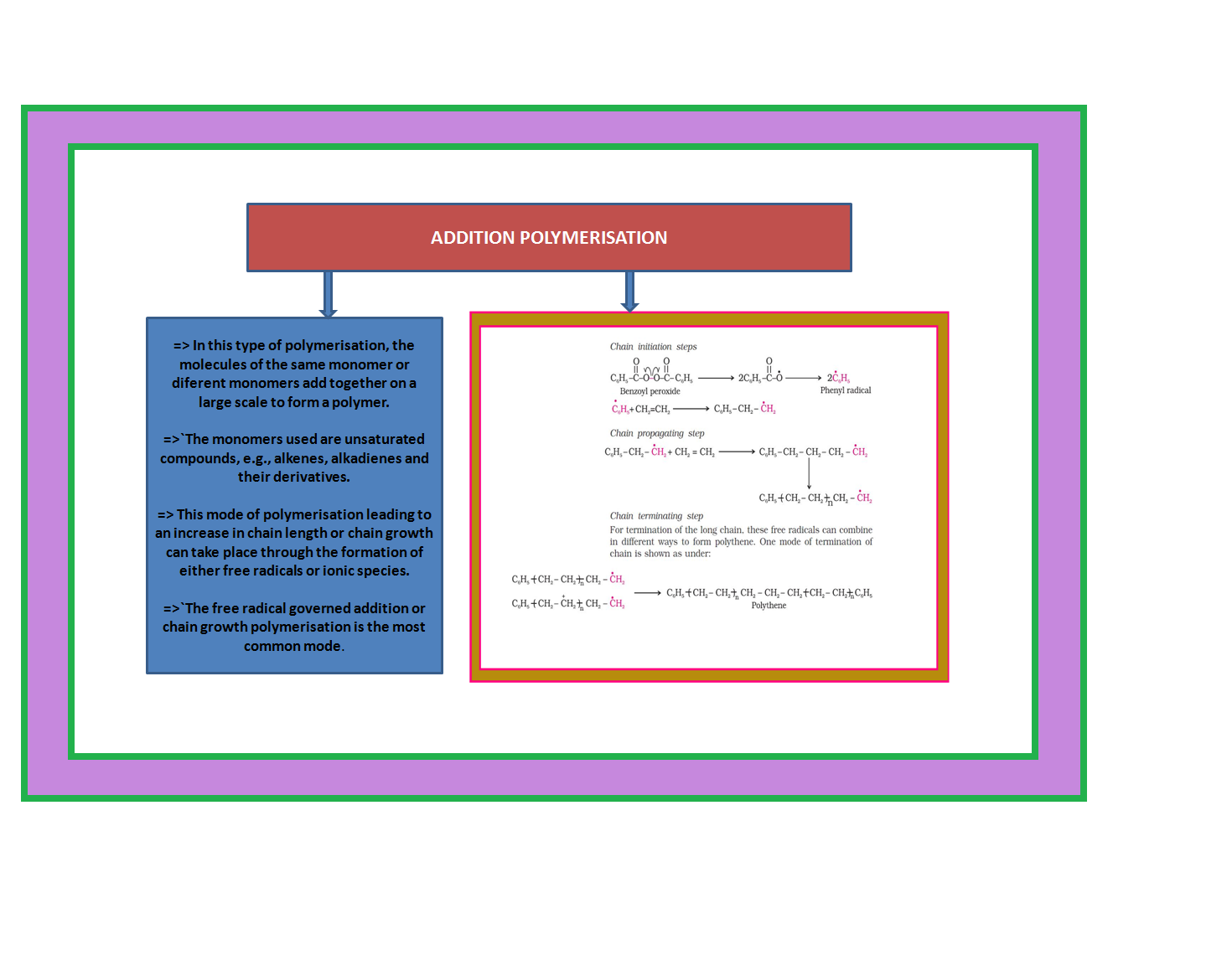
• `color{green}("Addition Polymers ")`: The addition polymers are formed by the repeated addition of monomer molecules possessing double or triple bonds.
`color{green}("Homopolymer ")`: The addition polymers formed by the polymerisation of a single monomeric species are known as homopolymers, e.g., polythene.
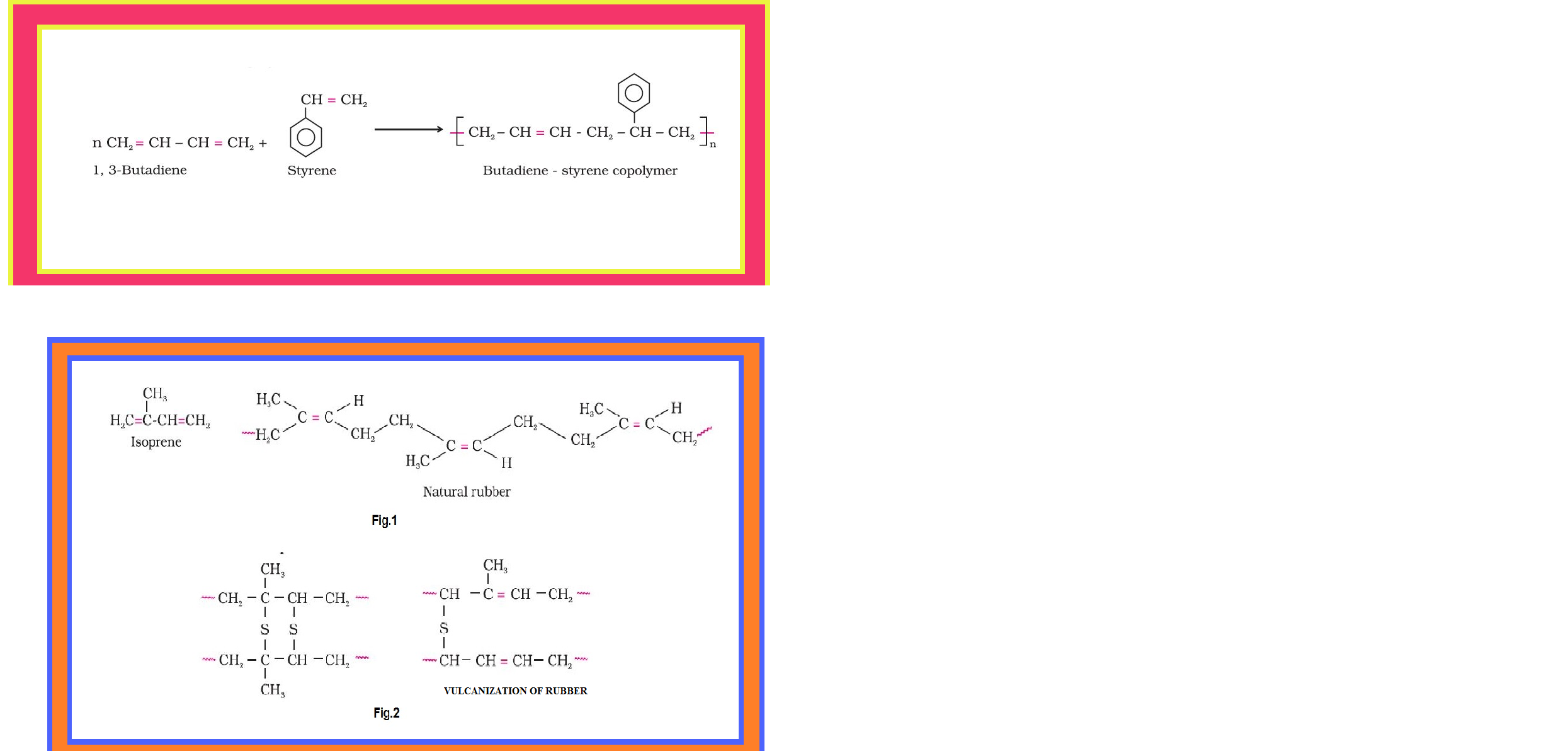
•`color{green}("Copolymer ")` : The polymers made by addition polymerisation from two different monomers are termed as copolymers, e.g., Buna-S, Buna-N, etc.
• `color{green}("Condensation Polymers ")` : The condensation polymers are formed by repeated condensation reaction between two different bi-functional or tri-functional monomeric units.
• `color{green}("Natural Polymers ")` : These polymers are found in plants and animals. Examples are proteins, cellulose, starch, resins and rubber.
• `color{green}("Semi-synthetic Polymers ")` : Cellulose derivatives as cellulose acetate (rayon) and cellulose nitrate, etc. are the usual examples of this sub category.
• `color{green}("Synthetic Polymers ")` A variety of synthetic polymers as plastic (polythene), synthetic fibres (nylon 6, 6) and synthetic rubbers (Buna-S) are examples of man made polymers extensively used in daily life as well as in industry.
•`color{green}("Linear Polymers ")` : These polymers consist of long and straight chains. The examples are high density polythene, polyvinyl chloride, etc. These are represented as shown in fig.1.
•`color{green}("Branched Chain Polymers ")` : These polymers contain linear chains having some branches, e.g. low density polythene.
•`color{green}("Cross Linked or Network Polymers ")` : These are usually formed from bi-functional and tri-functional monomers and contain strong covalent bonds between various linear polymer chains, e.g. bakelite, melamine, etc.
• `color{green}("Addition Polymers ")`: The addition polymers are formed by the repeated addition of monomer molecules possessing double or triple bonds.
`color{green}("Homopolymer ")`: The addition polymers formed by the polymerisation of a single monomeric species are known as homopolymers, e.g., polythene.
•`color{green}("Copolymer ")` : The polymers made by addition polymerisation from two different monomers are termed as copolymers, e.g., Buna-S, Buna-N, etc.
• `color{green}("Condensation Polymers ")` : The condensation polymers are formed by repeated condensation reaction between two different bi-functional or tri-functional monomeric units.